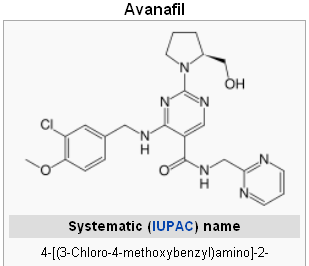
CONTRAINDICATIONS Stendra (Avanafil)
Administration of STENDRA with any style of organic nitrates, either regularly and/or intermittently, is contraindicated. In step with its known effects on the n . o ./cyclic guanosine monophosphate (cGMP) pathway, STENDRA can potentiate the hypotensive effects of nitrates.
Within a patient who's taken STENDRA, where nitrate administration is deemed medically necessary in the life-threatening situation, a minimum of 12 hours should elapse following your last dose of STENDRA before nitrate administration is considered. In such circumstances, nitrates should only be administered under close medical supervision with appropriate hemodynamic monitoring [see Contraindications , Dosage and Administration , and Clinical Pharmacology ].
STENDRA is contraindicated in patients that has a known hypersensitivity to any portion of the tablet. Hypersensitivity reactions are already reported, including pruritis and eyelid swelling.
Evaluation of erection problems (ED) ought to include the right medical assessment to recognize potential underlying causes, in addition to treatments.
Before prescribing STENDRA, you have to note the examples below:
There's a simple prospects for cardiac risk during sexual activity in patients with pre-existing coronary disease. Therefore, treatments for ED, including STENDRA, ought not to be employed in men to whom sexual activity is inadvisable greatly assist underlying cardiovascular status.
Patients with left ventricular outflow obstruction (e.g., aortic stenosis, idiopathic hypertrophic subaortic stenosis) the ones with severely impaired autonomic control over blood pressure level is usually particularly understanding of what of vasodilators, including STENDRA.
The next groups of patients just weren't used in clinical safety and efficacy trials for STENDRA, and as a consequence until further information is available, STENDRA is just not appropriate this groups:
Patients who have suffered a myocardial infarction, stroke, life-threatening arrhythmia, or coronary revascularization in the last few months;
Patients with resting hypotension (blood pressure below 90/50 mmHg) or hypertension (high blood pressure higher than 170/100 mmHg);
Patients with unstable angina, angina with intercourse, or Big apple Heart Association Class 2 or greater congestive coronary failure.
Like with other PDE5 inhibitors STENDRA has systemic vasodilatory properties and could augment hypertension-lowering effect of other anti-hypertensive medications. STENDRA 200 mg resulted in transient decreases in sitting blood pressure in healthy volunteers of 8.0 mmHg systolic and 3.3 mmHg diastolic [see Clinical Pharmacology (12.2)], using the maximum decrease observed at an hour after dosing. Even though this normally could well be expected to get of little consequence in most patients, prior to prescribing STENDRA, physicians should carefully consider whether patients with underlying heart disease may be affected adversely by such vasodilatory effects, specifically in combination with sexual activity.
STENDRA metabolism is especially mediated by the CYP 450 isoform 3A4 (CYP 3A4). Inhibitors of CYP 3A4 may reduce STENDRA clearance and increase plasma concentrations of avanafil.
For patients taking concomitant strong CYP3A4 inhibitors (including ketoconazole, ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir and telithromycin), do not use STENDRA [see Drug Interactions ].
For patients taking concomitant moderate CYP3A4 inhibitors (including erythromycin, amprenavir, aprepitant, diltiazem, fluconazole, fosamprenavir, and verapamil), maximum recommended dose of STENDRA is 50 mg, not to exceed once every a day [see Drug Interactions ].
Prolonged erection greater than 4 hours and priapism (painful erections over six hours in duration) have been reported compared to other PDE5 inhibitors. In the case connected with an erection that persists longer than 4 hours, the sufferer should seek immediate medical assistance. Or treated immediately, penile tissue damage and permanent lack of potency could result.
STENDRA need to be combined with caution in patients with anatomical deformation with the penis (like angulation, cavernosal fibrosis, or Peyronie’s disease), or perhaps patients that have conditions which might predispose these phones priapism (such as sickle cell anemia, multiple myeloma, or leukemia).
Physicians should advise patients to avoid utilization of all PDE5 inhibitors, including STENDRA and seek medical attention in the event of a sudden lack of vision in one or both eyes. This kind of event can be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a contributing factor to decreased vision including permanent decrease of vision which has been reported rarely postmarketing in temporal association with the aid of all PDE5 inhibitors. It is not possible to ascertain whether these events are associated right to the utilization of PDE5 inhibitors as well as to elements. Physicians should likewise discuss with patients the increased risk of NAION in individuals who have formerly experienced NAION in a eye, including whether such individuals may very well be adversely suffering from using vasodilators, for instance PDE5 inhibitors [see Adverse Reactions ].
Patients with known hereditary degenerative retinal disorders, including retintis pigmentosa, just weren't as part of the clinical trials of STENDRA, and employ in these patients is not recommended. Page 4 of 22
Using PDE5 inhibitors is regarding sudden decrease or loss in hearing, which can be accompanied by tinnitus or dizziness. It isn't possible to find out whether these events are associated straight away to the employment of PDE5 inhibitors or even additional circumstances [see Side effects (6)]. Patients experiencing these symptoms ought to be advised to end taking STENDRA and seek prompt medical attention.
Physicians should check with patients the possibility for STENDRA to augment the blood pressure-lowering effect of alpha-blockers and other antihypertensive medications [see Drug Interactions and Clinical Pharmacology ].
Caution is recommended when PDE5 inhibitors are co-administered with alpha-blockers. Phosphodiesterase type 5 inhibitors, including STENDRA, and alpha-adrenergic blocking agents tend to be vasodilators with hypertension-lowering effects. When vasodilators utilized in combination, an additive effect on high blood pressure could possibly be anticipated. In some patients, concomitant using the above drug classes can lower blood pressure significantly resulting in symptomatic hypotension (e.g., dizziness, lightheadedness, fainting).
Patients need to be stable on alpha-blocker therapy previous to initiating treatment which has a PDE5 inhibitor. Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant using PDE5 inhibitors.
In those patients who definitely are stable on alpha-blocker therapy, PDE5 inhibitors ought to be initiated at budget friendly dose (STENDRA 50 mg).
In those patients already taking an optimized dose of a PDE5 inhibitor, alpha-blocker therapy should be initiated at the smallest dose. Stepwise increase in alpha-blocker dose may perhaps be involving further lowering of high blood pressure when choosing a PDE5 inhibitor.
Safety of combined utilization of PDE5 inhibitors and alpha-blockers might be troubled by other variables, including intravascular volume depletion along with anti-hypertensive drugs [see Dosage and Administration (2) and Drug Interactions (7.1)].
Patients should be made aware that both alcohol and PDE5 inhibitors including STENDRA behave as vasodilators. When vasodilators are used combination, blood-pressure-lowering effects of every individual compound may perhaps be increased. Therefore, physicians should inform patients that substantial consumption of alcohol (e.g., above 3 units) when combined with STENDRA might increase the prospect of orthostatic signs, including surge in beats per minute, lessing of standing high blood pressure, dizziness, and headache [see Drug Interactions and Clinical Pharmacology ].
In conjunction with Other PDE5 Inhibitors or Erectile Dysfunction Therapies
The protection and efficacy of mixtures of STENDRA along with other treatments for ED has not been studied. Therefore, the utilization of such combinations just isn't recommended.
The security of STENDRA is unknown in patients with bleeding disorders and patients with active peptic ulceration. Ex vivo studies with human platelets indicate that STENDRA potentiates the anti-aggregatory effect of sodium nitroprusside (a nitric oxide supplements [NO] donor).
Using STENDRA offers no protection against sexually transmitted diseases. Counseling patients about the protective measures important to guard against std's, including HIV (HIV), should be thought about.